According to the Ministry of Food and Drug Safety on the 3rd, Onconic Therapeutics’ 'JAQBO' tablet and Vivozon Pharma’s 'Anapraju' were designated as the 37th and 38th domestically developed new drugs last year. This achievement comes two years after Daewoong Pharmaceutical’s Enblo was recognized as a new drug in 2022.
This shift has significantly boosted Yuhan’s financial performance. The company’s annual revenue surpassed KRW 2 trillion last year, making it the first South Korean pharmaceutical firm to enter the 'KRW 2 Trillion Club.' Its revenue increased by 11.2% from KRW 1.859 trillion in 2023 to KRW 2.0678 trillion in 2024.
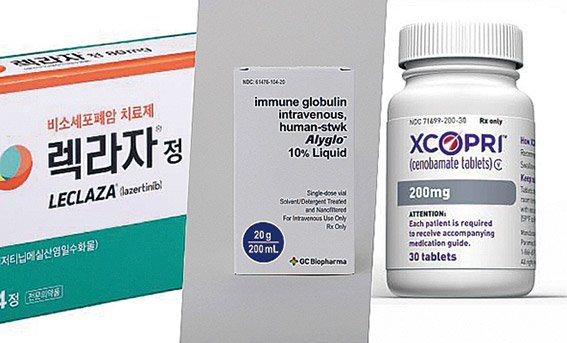
GC Biopharma also saw a major breakthrough. In December 2023, it secured FDA approval for 'Alyglo,' a treatment for immunodeficiency disorders, after three attempts since 2015. Since August last year, the company has been directly selling Alyglo through its U.S. subsidiary, GC Biopharma USA.
'Alyglo' contributed to the company’s expansion, generating KRW 48 billion in revenue in the fourth quarter alone. GC Biopharma’s total consolidated revenue in 2024 reached KRW 1.6799 trillion, a 3.3% increase from the previous year. The company expects Alyglo to generate annual sales of USD 300 million (approximately KRW 410 billion) by 2028.
SK Biopharm has also been making strides in the global market. Since obtaining FDA approval in 2019, the company has been directly selling its epilepsy new drug cenobamate under the brand name 'Xcopri' in the U.S. The drug, developed entirely in-house over a decade, has seen substantial growth in market presence.
In 2024, cenobamate’s prescription numbers in the U.S. surged. Monthly prescriptions rose from 26,283 in January to 35,516 in December, reflecting a 35.1% increase. Revenue also climbed sharply, from KRW 90.9 billion in Q1 to KRW 129.3 billion in Q4, marking a 42.2% jump. Since Q2 2024, Xcopri’s revenue has exceeded SK Biopharm’s total selling, general, and administrative (SG&A) expenses, driving overall profitability.
The Challenge of Developing the Next Pipeline
The challenge now is securing the next successful drug. Patent protection for new drugs typically lasts about a decade. Once patents expire, generic competition emerges, making sustained revenue growth difficult. This underscores the importance of preparing successor drugs well in advance, as the entire process—from discovering candidate substances to clinical trials and regulatory approvals—takes an average of 10 to 15 years.To address this, Yuhan Corporation is following the same strategy that led to Leclaza’s success. It is leveraging open innovation by licensing promising candidate substances from biotech firms, conducting early-stage clinical trials, and subsequently out-licensing them to global pharmaceutical giants. Leclaza itself was developed using a candidate substance sourced from Oscotec and later licensed to Johnson & Johnson’s Janssen division.
The company is currently exploring various candidate substances, with the most promising being YH35324, an allergy treatment. YH35324, which Yuhan acquired from GI Innovation, is undergoing early clinical trials. It has demonstrated superior therapeutic efficacy compared to existing allergy treatments, attracting interest from multiple multinational pharmaceutical firms for potential technology transfer agreements.
GC Biopharma is also accelerating efforts to develop the next Alyglo. The company is adopting a strategy of collaborating with pharmaceutical firms that possess strong R&D capabilities.
One prominent example is its partnership with Hanmi Pharmaceutical to develop 'LA-GLA (GC1134A),' an innovative treatment for Fabry disease. LA-GLA has been recognized for its efficacy and convenience, making it a closely watched product by regulatory agencies worldwide. The FDA approved its Phase 1/2 clinical trials in August 2024, and South Korea’s Ministry of Food and Drug Safety granted approval for local trials in January this year.
SK Biopharm, on the other hand, is utilizing artificial intelligence (AI) technology in its search for the next breakthrough drug. The company is leveraging its AI-based drug research and development platform, 'Hubble Plus,' to identify potential successors to cenobamate.
Hubble Plus is designed to enhance efficiency in various areas of new drug R&D, including radiopharmaceuticals (RPT) and targeted protein degradation (TPD). In June 2024, SK Biopharm recruited AI drug development expert Dr. Shin Bong-keun to further refine its AI-driven approach.
Lee Dong-hoon, CEO of SK Biopharm, stated, "We have been contemplating the use of AI technology for a much longer period than is publicly known. By leveraging Dr. Shin’s expertise and experience, we aim to enhance SK Biopharm’s existing AI-driven drug development platform and accelerate our digital healthcare initiatives.”
Kim Nayoung, Korea Finacial Times (steaming@fntimes.com)
[관련기사]
- Pharmaceutical DNA in Kolmar Korea and COSMAX... True Commitment to R&D
- Yuhan Corporation Becomes First Korean Pharma to Join the '2 Trillion Won Club'—Will There Be a 'Next Leclaza' This Year?
- Samsung Biologics Replaces CFO and R&D Head... Will It Find New Momentum This Year?
- K-PharmaBio’s First 'KRW 4 Trillion Club' Member – Samsung Biologics Eyes KRW 5 Trillion with Unmatched Excellence in Capacity
- 'COVID-19 bad times over' Youngjin Pharmaceuticals' operating profit soars 180% last year...'What's the secret to its rapid growth?
가장 핫한 경제 소식! 한국금융신문의 ‘추천뉴스’를 받아보세요~
데일리 금융경제뉴스 Copyright ⓒ 한국금융신문 & FNTIMES.com
저작권법에 의거 상업적 목적의 무단 전재, 복사, 배포 금지












![Samsung Electronics and SK Hynix Engage in High-Stakes AI Chip War [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=284&h=214&m=5&simg=2026013013432407964141825007d12411124362.jpg&nmt=18)
![Hyundai Mobis Posts Record Results, Pivots to Robotics and Electrification [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=284&h=214&m=5&simg=2026012911595100522141825007d12411124362.jpg&nmt=18)
![One Year In: CEO Hyun Shin-kyun Transforms LG CNS into KRW 6 Trillion AI Powerhouse [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=284&h=214&m=5&simg=2026012807381208149141825007d12411124362.jpg&nmt=18)
!['Chung Eui-sun Declares Robot Management Era'... Hyundai Motor Group to Lead Robotics Age with 'Physical AI' [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=284&h=214&m=5&simg=20260106111449071567492587736121125197123.jpg&nmt=18)
![Lee Jae-yong and Chey Tae-won, Korea's 'Semiconductor Duo,' Head to China... What's the Status of Local Operations? [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=284&h=214&m=5&simg=2026010608232205705141825007d12411124362.jpg&nmt=18)
!['Beyond Mobile to Robotics': LG Innotek Continues Portfolio Expansion [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=284&h=214&m=5&simg=2026010508201109906141825007d12411124362.jpg&nmt=18)
![Doosan Enerbility Eyes Return to 'A' Credit Rating After 9 Years on Nuclear Power and Gas Turbine Boom [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=284&h=214&m=5&simg=2026010208511504157141825007d12411124362.jpg&nmt=18)
!["Samsung Is Back," Chip Chief Declares, Eyeing Record Profit on HBM Recovery [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=110&h=79&m=5&simg=2026011408580109459141825007d12411124362.jpg&nmt=18)
![Koo Kwang-mo's Vision for AI and Robotics Drives LG Group's Transformation [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=110&h=79&m=5&simg=2026012123034202714141825007d122461258.jpg&nmt=18)
!['Expansion' S-OIL vs. 'Caution' GS Caltex [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=110&h=79&m=5&simg=2026012010260905723141825007d12411124362.jpg&nmt=18)
![One Year In: CEO Hyun Shin-kyun Transforms LG CNS into KRW 6 Trillion AI Powerhouse [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=110&h=79&m=5&simg=2026012807381208149141825007d12411124362.jpg&nmt=18)
![Samsung's 'Last Chance': Lee Jae-yong Pushes HBM4 as Make-or-Break Moment [KFT Topic]](https://cfnimage.commutil.kr/phpwas/restmb_setimgmake.php?pp=006&w=110&h=79&m=5&simg=2026012623242806011141825007d122461258.jpg&nmt=18)